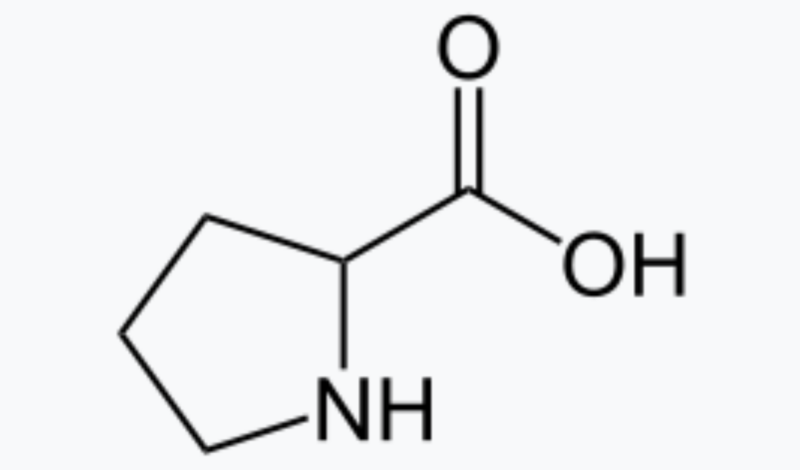
Enlarge / Proline is a common amino acid. It’s also an effective catalyst. (credit: Wikimedia Commons )
Platinum is a ferociously expensive metal that is difficult to obtain and purify. Most of the small supply we produce every year isn’t put to use for its properties as a metal. Instead, it’s used as a catalyst for producing a variety of chemicals—and for cleaning up your car’s exhaust. Everything made with platinum carries an added burden of cost and environmental damage because of that use.
This year, the Nobel Prize in Chemistry honors two researchers for helping to trigger a burst of research into catalysts that leave the metals behind. Benjamin List and David MacMillan made key discoveries that started the field of organocatalysis, developing catalysts that could be made from cheap, common chemicals. Their work took a disorganized set of anecdotes and gave them a strong conceptual footing that allowed many other labs to go further.
Not so metal
At their heart, chemical reactions involve the transfer of electrons, either between atoms or into new configurations of chemical bonds. Metals are often effective catalysts because they ease the process of transferring electrons. Many metals will easily make a temporary loan of their electrons during a reaction or, if properly prepared, can draw electrons from other chemicals in order to hasten a process.
