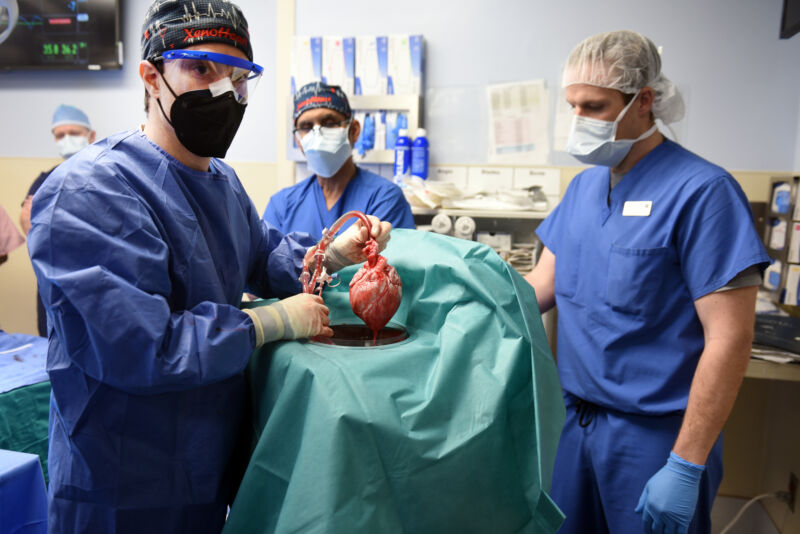
Enlarge / The transplant team with the replacement heart. (credit: The transplant team with the replacement heart.)
On Monday, the University of Maryland School of Medicine announced that its staff had completed the first transplant of a pig’s heart into a human. The patient who received the heart had end-stage heart disease and was too sick to qualify for the standard transplant list. Three days after the procedure, the patient was still alive.
The idea of using non-human organs as replacements for damaged human ones—called xenotransplantation—has a long history, inspired by the fact that there are more people on organ waiting lists than there are donors. And in recent years, our ability to do targeted gene editing has motivated researchers to start genetically modifying pigs in order to make them better donors. But the recent surgery wasn’t part of a clinical trial, so it shouldn’t be viewed as an indication that this approach is ready for widespread safety and efficacy testing.
Instead, the surgery was authorized by the Food and Drug Administration under its “compassionate use” access program, which allows patients facing life-threatening illnesses to receive investigational treatments that haven’t gone through rigorous clinical testing yet.
